

Each vertical column on the periodic table is called a group.As you move from left to right across a period, elements transition from displaying metal characteristics toward nonmetallic properties. Elements in the same period all have the same electron ground state energy level. There are seven periods on the periodic table. Each horizontal row on the periodic table is called a period.The original table organized the elements by increasing atomic weight. This is the biggest difference between today's periodic table and Mendeleev's periodic table. Every atom of element 118 has 118 protons. Until a new element is discovered, the last element on the table is element number 118. So element number 1 (hydrogen) is the first element. The atomic number is the number of protons in an atom of that element. Elements are listed in numerical order by atomic number.“Chemistry for Non-Majors.” Lumen, Lumen Learning. “The Difference Between an Element Group and Period.” ThoughtCo, Aug. And, the key difference between periods and groups is that periods are horizontal rows whereas groups are the vertical columns in the periodic table of chemical elements. To sum up what we discussed here, periods and groups are two ways that we categorize the chemical elements in the periodic table. That is, the elements in the same period have the same number of electron shells while the elements in the same group have the same number of valence electrons. Furthermore, we can also find another difference between periods and groups in the eletron arrangement. There are 7 major periods and 18 groups in the periodic table of elements. Hence, this is the key difference between periods and groups of the periodic table. Periods are the horizontal rows in the periodic table whereas groups are the vertical columns in the periodic table. What is the Difference Between Periods and Groups in Periodic Table? For example, we call the group 1 element as alkali metals and group 2 elements as alkali earth metals. However, some groups have common names as well.
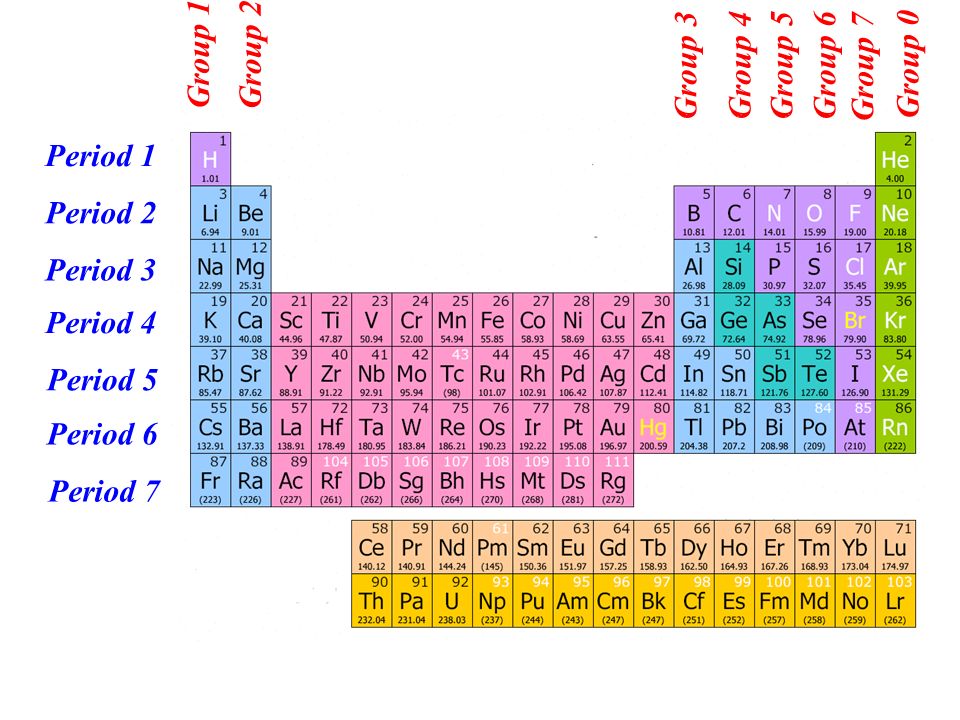
There are mainly 18 groups in the periodic table. Most of the times, the elements in the same group shares somewhat similar chemical and physical properties. For example, the number of valence electrons in the group 1 is 1. All the chemical elements in the same group contain an equal number of valence electrons. Groups are the vertical columns in the periodic table. Thus, we name them as period 1, period 2, … Period 7. Mainly, there are 7 periods in the periodic table. This is because, the number of elements is determined by the number of electrons allowed in each electron shell. However, the periods have different numbers of members there are more chemical elements in some periods than some other periods. Moreover, the elements get less metallic when we move forward a row. When we go through a row, the number of protons in the atomic nucleus increases by 1 for each next element in the period. All the chemical elements in this row have the same number of electron shells. Periods are the horizontal rows in the periodic table. Summary What are Periods in Periodic Table? Side by Side Comparison – Periods vs Groups in Tabular Formĥ. Further, each row and column has specific characteristics, and we name a row as a period and a column as a group in the periodic table. Hence, as with any grid, this periodic table also has rows and columns. The periodic table of elements is a large table in which each and every known chemical element is placed in a specific location considering the atomic structures. The key difference between periods and groups is that the periods are horizontal rows whereas the groups are the vertical columns in the periodic table of chemical elements.


 0 kommentar(er)
0 kommentar(er)
